Monsoon moisture continues and temperature changes for the valley's
Introduction
Due to differences in h2o quality and combination (H2 16O, HDO, H2 18O, and H2 17O), isotope fractionation occurs in stage transformation under different meteorological conditions. Therefore, differences in the content of hydrogen and oxygen stable isotopes in a water trunk reflect its physical characteristics, such as water evaporation and manual during the water bike. Water isotopes are widely used as tracking indicators in paleoclimate reconstruction and water cycle research (Bolin, 1959; Ren et al., 2016). Recorded historical details concerning changes in the water cycle process can track the migration and condensation process of h2o vapor by monitoring atmospheric h2o vapor and atmospheric precipitation isotopes (Dong et al., 2017; Zhang et al., 2020).
In the past, there take been very few studies related to water vapor-stable isotopes and were based on discrete water vapor samples collected past traditional cryogenic cold trap techniques (Vuille et al., 2005). These instruments limit the power to find and collect long-term and high-resolution atmospheric water vapor-stable isotope data. Recent in situ measurements using spectroscopic techniques have made it possible to monitor continuous changes in atmospheric water vapor-stable isotopes on finer timescales. Over the past decade, this new technique has been used to monitor stable isotopes of h2o vapor in unlike climate zones around the world. The hydrogen- and oxygen-stable isotopes of atmospheric water vapor research have progressed from early qualitative to the electric current quantitative and model descriptions. Eriksson (1965) used an isotope model based on the atmospheric water vapor circulation model and establish that δeighteenO and δiiH in atmospheric water vapor decreased exponentially with altitude variation. Contempo studies have systematically described the distribution of stable isotopes of atmospheric water vapor on a iii-dimensional spatial level in some regions and analyzed the seasonal changes in water vapor isotopes based on a nine-year about-surface atmospheric h2o vapor isotope data in the eastern Mediterranean (Angert et al., 2008; Rozanski and Sonntag, 2010). Steen-Larsen et al. (2011) plant a substantial correlation between the atmospheric h2o vapor isotopes of Greenland and the relative near-surface humidity. Steen-Larsen et al. (2014) establish that the daily changes of atmospheric water vapor isotopes were strongly correlated with the relative humidity, and the seasonal variation is related to weather weather condition. COSMOiso is an isotope-enhanced regional model that was used to investigate the influence of continental evapotranspiration, precipitation, and sub-cloud processes on the δ2H of meteoric water vapor and precipitation in Europe. Under warm conditions, continental evapotranspiration, atmospheric precipitation, and sub-cloud processes at constant temperatures can command the effects of near-surface water vapor (Christner et al., 2018). In Lhasa, h2o vapors δ18O and δtwoH were compared with concurrent daily precipitation isotopes to assess the seasonal control of vapor isotopes and possible correlations with precipitation isotopes. Atmospheric water vapor δ18O has intra-seasonal dependence on a large-scale meteorological government, and the seasonal variation of vapor d-excess is weak. During the summer monsoon flavour, high-frequency fluctuations of both δxviiiO and d-excess persisted for several days, and these fluctuations are in phase with precipitation events, confirming the pregnant effect of local evaporation on the short-term water wheel (Tian et al., 2019).
Some studies have analyzed the stable isotopes of hydrogen and oxygen in the arid and semiarid regions of China. The temperature issue, which primarily occurs in middle and high latitudes, is from coastal regions to inland continents, and the positive correlation is closer. However, there are many negative correlations in the middle and low latitudes and in the southern Qinghai–Tibet Plateau (Zhang and Yao, 1998; Liu et al., 2002). Moreover, the seasonal variation of δeighteenO indicates that westerly water vapor, local moisture, and summer monsoon all take a greater affect on the region. Among these, westerly water vapor plays a dominant role (Chen et al., 2013). In the northern monsoon region, precipitation isotope values are primarily affected by secondary evaporation. During the transportation process, leaching action increases the degree of precipitation isotope fractionation, and stalagmite oxygen isotope records from the northern monsoon region can reflect changes in local annual precipitation (Zhao, 2018). Using field observations of δ2H and δ18O in atmospheric h2o vapor over bogus haven farmland in the Heihe River Bowl, it was constitute that local evapotranspiration, particularly transpiration, is conducive to the enrichment of heavy isotopes in surface h2o vapor, and boundary layer entrainment can deplete heavy isotopes in surface water vapor (Huang and Wen, 2015).
Some global climate and hydrological process studies based on 17O and other stable isotopes have achieved various results. The 17O-excess originates from the evaporation of seawater into marine air, and from the transfer of atmospheric vapor to liquid or solid precipitation, and high 17O-excess in meteoric water is indicative of oceanic origin, where air relative humidity is low, and vice versa. A significant decrease in 17O-backlog occurs when a reservoir of meteoric water is subjected to excessive evaporation (Luz and Barkan, 2010). In Antarctica, 17O-backlog was measured and theoretically calculated using the Rayleigh fractionation model. To explain air quality, information technology was proposed that the heterogeneity of 17O-excess in precipitation in Antarctica can exist explained not simply by the temperature at the time of condensation or the relative humidity of the moisture source expanse simply also by the difference in moisture source and the difference in supersaturation along the line. The continuous precipitation procedure will cause the δ17O value in precipitation to gradually refuse, that is, show the leaching effect. The relationship between δfifteenNorth, δeighteenO, and δ17O in nitrates in the snow cannot be explained only by the local postal service-precipitation process but should be explained in the context of the main atmospheric signals (He et al., 2015; Pang et al., 2015; Shi et al., 2015). In an ice-covered sea nearly Syowa Station, the isotopic variation was found to exist related to a change in large-scale circulation caused by the trough–ridge–trough pattern. Its range reflects the littoral-inland movement of the front between the warm maritime air mass enriched past the δ2H value and common cold glacial air mass depleted past the δtwoH value (Kurita et al., 2016).
In previous studies on stable isotopes of hydrogen and oxygen, 17O has been neglected because of its limited applications. More recently, with scientific and technological advancements, research on the outcome of isotope fractionation and the loftier-precision measurement of 17O has evolved (Ma et al., 2010). It also provided more methods for studying the relationship between oxygen isotopes and geographical factors, such as the water bicycle and the origination of moisture. However, studies on meteoric water vapor 17O in the barren and semiarid regions of China are scant.
In this written report, nosotros conducted a field experiment to collect real-time isotope information on the nigh-surface atmospheric water vapor from May to November 2019 in Lanzhou, a typical semiarid region. Our objectives were to place the moisture sources and analyze the influences of regional meteorological conditions on vapor isotope variations at diurnal and seasonal timescales. This will further our understanding of the characteristics of atmospheric water vapor-stable isotope compositions in semiarid regions and provide insights into singled-out moisture sources and local atmospheric and hydrological processes.
Materials and Methods
Site Description
The on-site observations were conducted in a semiarid valley of the Yellow River in northwest inland China (Figure i), which is a topography of the valley sandwiched between northern and southern mountains. Information technology is located in the transition zone from the Qinghai–Tibet Plateau to the Loess Plateau. The Lanzhou river valley has a continental semiarid climate, with four singled-out seasons. Hot and rainy summers are influenced by the east asian summer monsoon, and cold and dry winters are dominated by the northward polar air mass. The average almanac temperature is approximately xi.2°C, and the average almanac precipitation is 367.9 mm, of which lxx% occurs during the summertime season of June–September. Lanzhou is located on the fringe of Prc's monsoon area (Zhao, 1983) and belongs to the transition area between the westerlies and summer monsoon. The temporal and spatial distribution of atmospheric water vapor and precipitation in the monsoon fringe area is extremely uneven and highly variable. It is an important surface area reflecting the evolution of the monsoon and its regional influence. The monsoon edge region has typical environmental sensitivity and vulnerability. The study of climate modify in the monsoon edge region is of great significance for exploring the monsoon process, development machinery, and global environmental modify. Therefore, the meteoric water vapor and precipitation mechanisms in Lanzhou are complex and important.
FIGURE one
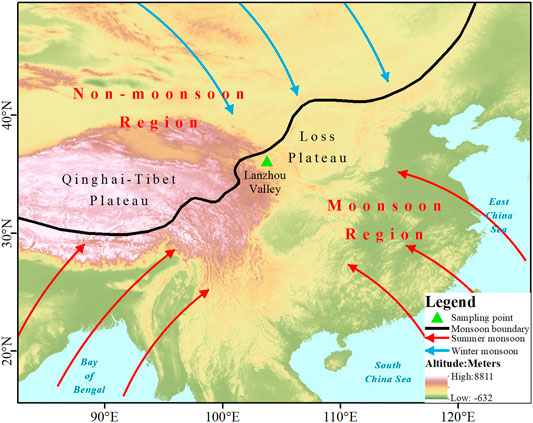
FIGURE 1. Location of the study area (Lanzhou valley) and sampling point characteristic by monsoon and westerly dual affection. The monsoon boundary was defined according to Zhao (1983).
Sampling and Assay
With the consideration of the continuity and timeliness of the water vapor observations, a site was selected in the Meteorological Park of the new campus of Northwest Normal University, where the influence of plant shrubs and human activities on the samples could be avoided. At that place were no loftier copse and shrubs in the vicinity of the sampling bespeak (Figure 2A), and the building was at a substantial altitude from the sampling equipment. From May to November 2019, an automatic weather station in the park was used to collect real-time solar radiation, air temperature, air relative humidity, wind speed, wind management, and precipitation information. To form an effective altitude correspondence, meteorological elements such as air temperature, relative air humidity, wind speed, and air current management twenty m above the ground at the automatic weather station were selected. Atmospheric water vapor and precipitation were measured in real time.
Figure 2
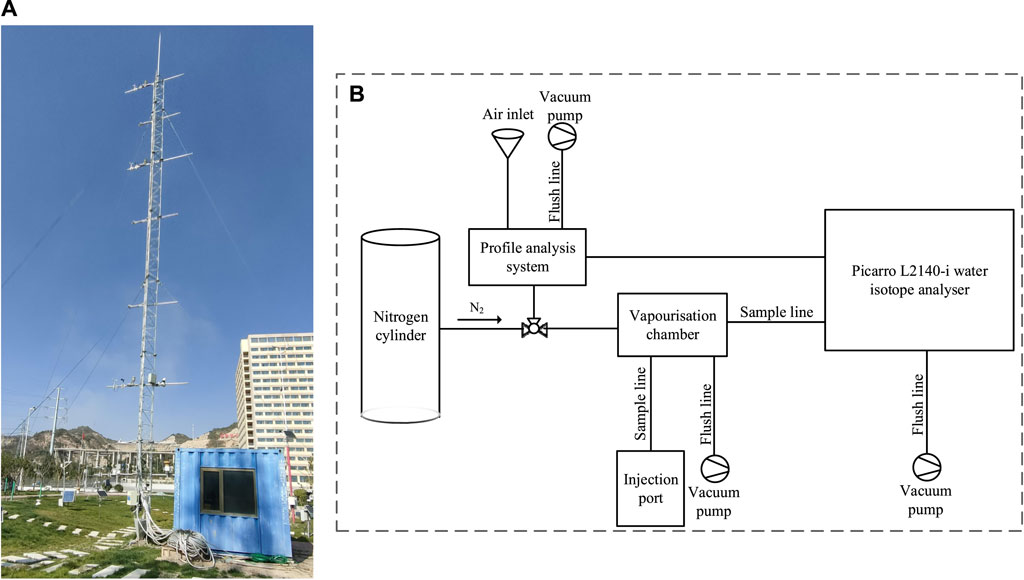
Effigy two. Sampling point environment and equipment system with (A) outdoor inlet tube tower and (B) indoor calibration and measuring instrument.
System Blueprint of Experimental Instrument
To address the uniqueness of existent-time long duration and observation, nosotros designed a h2o vapor and precipitation isotope ascertainment organization containing two parts. The kickoff part was composed of conditions towers of five-grand intervals and with four layers. Meteoric water vapor inlets, temperature and humidity monitors, anemometers, and wind direction meters were placed on each layer, with the precipitation measuring cylinder placed 2 thousand above the footing. The 2nd office was an L2140-i high-precision water isotope analyzer (Picarro, Inc., Santa Clara, United States) that measured δ2H, δ17O, and δ18O and was equipped with wavelength scanned crenel ring-down spectroscopy (WS-CRDS) (Figure 2B). Compared with the traditional continuous-flow isotope ratio mass spectrometry (CFIRMS), WS-CRDS has better accuracy in water isotope analysis and real-fourth dimension observation. The second part consisted of the following three components:
1) The contour analysis organization was the main body (Figure 2A). The air inlet transported atmospheric water vapor from ambient air to the profile analysis system through a copper pipe wrapped in blackness cream. The contour analysis system was flushed with an air pump and nitrogen.
2) The Picarro L2140-i water isotope analyzer (Figure 2B): In this process, atmospheric water vapor isotopes of hydrogen and oxygen were analyzed in the cavity. Closely following the analysis, the vacuum pump and nitrogen flushed the analyzing chamber to avoid remainder moisture affecting the next sample measurement.
3) The vaporization bedroom and nitrogen cylinder: The wiring of the vaporization chamber and nitrogen cylinder is shown in Figure 2B. This office was primarily used for standard sample testing. The needle drew a sure corporeality of standard water sample and injected it into the vaporization chamber. After vaporization, information technology entered the cavity for testing. After each standard water sample test was completed, nitrogen entered the vaporization sleeping room and cavity and was flushed with a vacuum pump to reduce experimental error.
Scale Protocol
The isotope determination from sampling, standard material packaging, storage, sample loading to standard textile choice, and sequence setting will impact the measurement results considering of the large variation in the abundance of hydrogen and oxygen isotopes in h2o bodies. This may lead to a large difference betwixt the measured and true values, and therefore, a correction of the measured values before the information analysis is required.
Two-Indicate Correction
According to different mathematical algorithms, the main standardization and correction of isotope data methods are the reference gas, one-point (using i standard substance), and two-indicate or multi-bespeak methods (using two or more standard substances). In this study, nosotros used the two-indicate method recommended by the International Diminutive Energy Agency (IAEA) and selected LGR-2E and LGR-5E as standard materials. The measured and true values of the hydrogen and oxygen isotope standard samples are listed in Table ane. The X-coordinate centrality was the measured value of the Picarro L2140-i isotope conclusion standard substance, and the Y-coordinate axis was the true value of the standard substance. A besprinkle diagram and tendency line could then be obtained. Past substituting the measured values of atmospheric water vapor and precipitation isotope into the linear equation, the truthful value of the sample in this experiment was obtained.
Tabular array ane
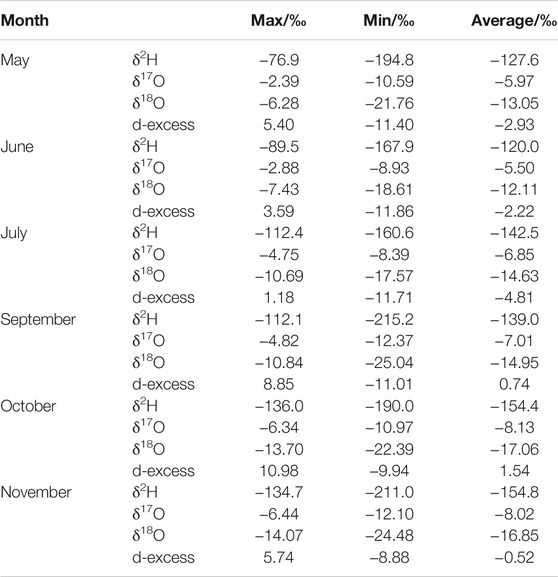
Tabular array 1. Summary of the atmospheric water vapor isotope monitoring information in Lanzhou.
The standard samples used were LGR-2E (δ17O = −8.79‰, δ18O = −16.71‰, and δtwoH = −123.8‰) and LGR-5E (δ17O = −1.52‰, δ18O = −two.99‰, and δtwoH = −9.9‰). A centralized standard sample measurement was established for two months to right the measured data of meteoric water vapor and atmospheric precipitation during this time menses.
All the samples were inputted into the American Picarro L2140-i water isotope analyzer in real time, which simultaneously measured δ18O, δ17O, and δ2H in liquid and gaseous water. We used the conventional δ notation to report isotope data (Craig, 1961; Merlivat and Jouzel, 1979):
where
In water apportionment, the departure in d-excess (Dansgaard, 1953; Dansgaard, 1964) is generated using the following formula:
The calculated d-backlog is primarily affected by the temperature, humidity, and current of air speed of the identify where the water originates from.
Humidity-Isotope Response Calibration
Each private analyzer needs to characterize the measured isotope value as a part of water vapor concentration (Aemisegger et al., 2012; Gu et al., 2019). Some changes are caused past atmospheric isotope indicate detection analyzers, for example, because of the temperature or humidity response, and therefore, it is necessary to thoroughly characterize the instrument system (Steen-Larsen et al., 2013). We applied the following calibration process (Bailey et al., 2015; Galewsky et al., 2016):
one) Ready the standard samples. The 2 standard samples used in this written report were LGR-2E (δ17O = −viii.79‰, δ18O = −16.71‰, and δ2H = −123.8‰) and LGR-5E (δ17O = −one.52‰, δxviiiO = −2.99‰, and δ2H = −9.nine‰).
2) Eliminate the memory effect. Picarro L2140-i data processing software (SDM Data Processor) was used to filter out the isotopic information of the standard samples and atmospheric water vapor and delete the offset 3 min of the atmospheric water vapor isotope data later on each measurement of LGR-2E and LGR-5E.
3) Calibrate instrument concentration effect. A water vapor concentration of twenty,000 ppmv was used as the benchmark, and information technology is assumed that the consequence of the isotope concentration is minimal nether this standard. The measured information of the meteorological tower do not contain the ppmv value of the atmospheric water vapor concentration, and therefore, it requires conversion:
where
where
4) Known standard calibration. The isotope measurement value of the standard sample every 2 months was used to found a linear relationship with its truthful value (see Section 2.five.ane).
five) Data standardization. Using the equation in step three, the corrected atmospheric water vapor concentration was used every bit the independent variable to obtain the h2o vapor hydrogen and oxygen isotope get-go values. The offset value was subtracted from the atmospheric water vapor isotope measurement result, and so, the linear equation in step iv generated the true value of the atmospheric water vapor hydrogen and oxygen stable isotopes (Steen-Larsen et al., 2014).
Results and Word
General Characteristics of Vapor Isotopic Compositions
The characteristic values of the temper vapor isotopes during 2019 summer including the maximum, minimum, and average values of δ2H, δ17O, δ18O, and d-excess are shown in Table 1. The δ18O values vary from −25.04‰ to −vi.28‰, with a mean value of −15.ten‰, and 90% of the values range from −21.74‰ to −8.54‰ throughout the survey flow (Table i and Figure iii). In comparison, the mean δeighteenO value is more positive than the annual average of −20.7‰ reported in Beijing (40°00′ North, 116°23′ E) (Wen et al., 2010), and it is more negative than the monthly average of -14.85‰ recorded in Zhangye (38°51′ Northward and 100°22′ Eastward) (Huang and Wen, 2015). When considering the geographical locations of the three stations, the difference between them reflects the influence of geographical factors on the h2o vapor isotopes to a sure extent, that is, the atmospheric water vapor isotopes gradually enrich from monsoon regions to non-monsoon regions in China. The hydrogen values δ2H vary from −215.2‰ to −76.9‰, with a mean value of −141.four‰, and 87% of the data were distributed between −174.4‰ and −105.four‰ in the survey menses. The atmospheric water vapor isotopes generally have a broad range of changes and maintain a downward trend from May to July, with an upward trend with a modest range of changes from July to September. They have a small range of changes and enter a stable state from September to November. The d-excess value more often than not maintains an upwardly tendency, but fluctuates often, peculiarly from May to September.
Figure 3
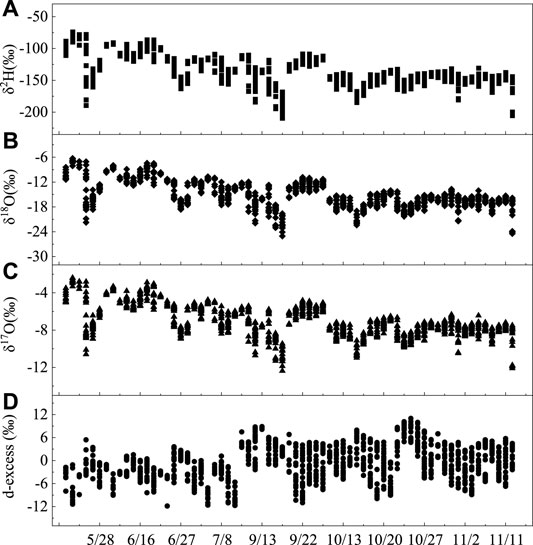
FIGURE 3. Daily mean δtwoH (A), δ18O (B), δ17O (C), and d-backlog (D) atmospheric water vapor variations well-nigh the surface during the entire sampling period in Lanzhou, China.
Diurnal Variations
Figure iv shows the 24-h ensemble average values of the h2o vapor isotopes. The dataset was divided into May, June, July, September, October, and Nov. The peak-to-height vapor δ18O variations were fifteen.48, 11.eighteen, vi.88, fourteen.20, 8.68%, and 10.41‰ in May, June, July, September, Oct, and November, respectively. The tiptop-to-peak vapor δ2H variations were 117.9, 78.v, 48.2, 103.1, and 54.0‰ in May, June, July, September, October, and November, respectively.
Effigy 4
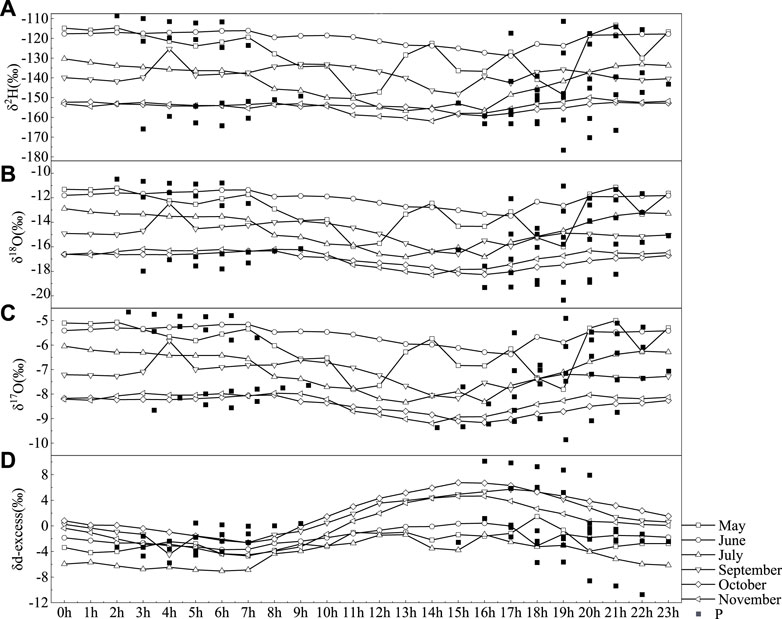
FIGURE 4. Hourly values of δ2H (A), δ18O (B), δ17O (C), and d-excess (D) of the atmospheric water vapor and atmospheric precipitation (squares) from May to November 2019 in Lanzhou, Prc, and 76.3‰, respectively. The minimum δiiH and δxviiiO values occurred in the afternoon (1400–1800 CST, Chinese standard fourth dimension), and the δ2H and δeighteenO maximums appeared at midnight. These diurnal variations appeared to be in stage with the vapor mixing ratio variations (Welp et al., 2008).
The d-excess value maintains a diurnal cyclic trend of depression–loftier–low day during the observation catamenia, and the maximum value generally appears between 15 and 18 h (Figure 4). The values of δ17O and δ18O from 0 to 10 h remain basically stable, while the values from 11 to 23 h fluctuate substantially over time.
Relationships Between Atmospheric Vapor Isotopes and Meteorological Factors on Unlike Timescales
Monthly and Intra-Month Variations of Daily Vapor Isotopic Compositions
The isotopes of atmospheric water vapor and precipitation gradually become positive equally the temperature increases and show a positive correlation. This phenomenon has a temperature effect (Tian et al., 1997). The changes in temperature, relative humidity, and precipitation (hereafter referred to as T, RH, and P) during the sampling period are shown in Effigy 5. The temperature begins to increase gradually in May, and then begins to decrease after reaching its highest value in July. The temperature drops significantly because of discontinuous information. In terms of relative humidity, considering of heavy precipitation in June, air humidity rose sharply then declined in July. Humidity began to increase in September, and and then decrease with the onset of winter.
FIGURE v
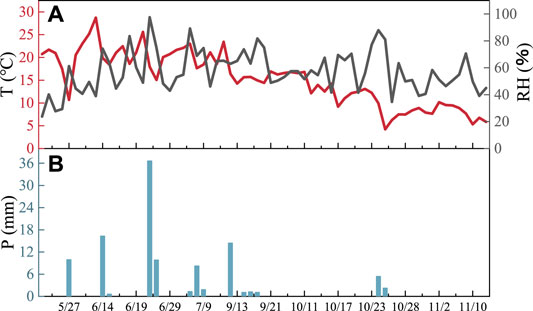
FIGURE v. Temperature, relative humidity (A), and atmospheric precipitation (B) during the sampling flow.
The temperature effect proposed by Dansgaard (1964) was used to analyze the human relationship between h2o isotopes and temperature. The daily scale correlation between the δ17O of atmospheric h2o vapor and temperature (Figure 6) is follows:
Effigy 6
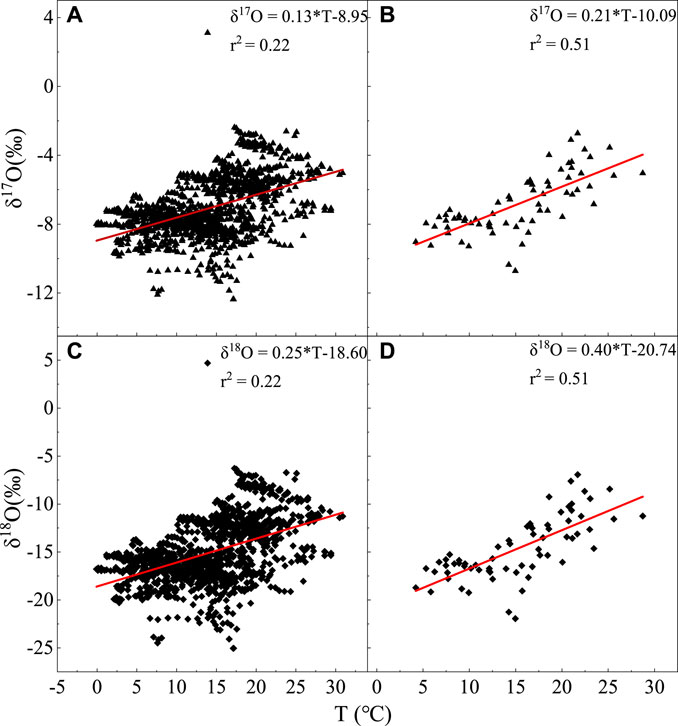
Figure half dozen. Relationship between δ17O and δ18O and temperature changes on daily and monthly scales, with (A) δ17O and daily scale, (B) δ17O and monthly scale, (C) δ18O and daily scale, and (D) δ18O and monthly scale.
The monthly-scale correlation shown in Effigy 6B is as follows:
In Figures 6C,D, the correlation between δ18O and temperature on daily and monthly scales is every bit follows:
and
This concords with the positive correlation of temperature furnishings, and the correlation is more significant. The temperature effect is because the fractionation of stable isotopes in atmospheric water vapor, and atmospheric precipitation is primarily governed by the temperature during phase transitions (Dansgaard, 1964; Rozanski et al., 1992; Zhang et al., 2003), and the modify in the surface temperature corresponds to a change in the condensation temperature of atmospheric precipitation cloud over the sky to a certain extent (Craig and Gordon, 1965). The lower the temperature, the greater the fractionation coefficient a of the isotopes in the h2o vapor and precipitation, and the lower the δ17O and δ18O in water vapor and atmospheric precipitation.
Diurnal Variations of Vapor Isotopic Compositions
This feature of daily variation is primarily related to evaporation and atmospheric turbulence in the study expanse. In the process of evaporation, the stable isotopic fractionation of hydrogen and oxygen will non exist produced (Xie et al., 2016). First, because of the absence of trees and shrubs near the experimental area, ground evaporation causes the hydrogen- and oxygen-stable isotope ratios in the atmospheric water vapor well-nigh the surface to be relatively high. The water vapor contributed by the underlying surface evaporation to the virtually-surface layer has a relatively high stable isotope ratio. Second, the strength of the atmospheric turbulence affects the mixing which is between high and low layers of the atmospheric water vapor isotopes. The air exchange betwixt the upper and lower layers was enhanced, and stable isotopes of hydrogen and oxygen in the atmospheric water vapor nigh the surface decreased when the turbulence increased. The change tendency of the stable isotopes of hydrogen and oxygen in the atmospheric water vapor near the surface became smaller when the turbulence became weaker. Therefore, afterwards sunrise on a clear day, the air temperature near the ground increased, the lower layer tended to become unstable, turbulent mixing was strengthened, and evaporation accelerated. Afterwards 11:00, the stable isotopes of hydrogen and oxygen in the virtually-surface h2o vapor continued to decrease as the water vapor content decreased. As the temperature began to subtract after 17:00, the turbulent effect weakened. At this time, the evaporation effect was greater than the turbulent effect, and the stable isotopes of hydrogen and oxygen in the near-surface water vapor began to increase slowly until 2:00 the adjacent twenty-four hour period (Figure 7).
Figure vii
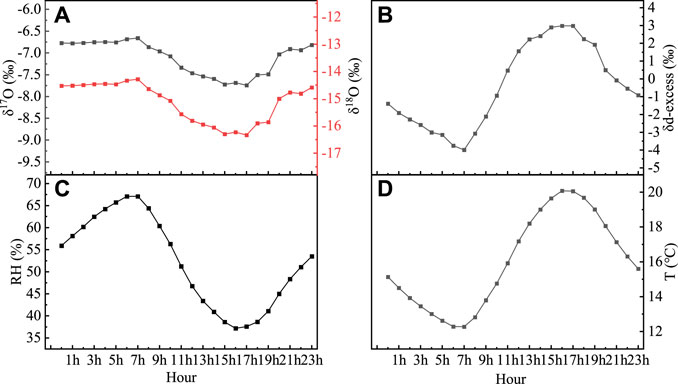
FIGURE 7. Daily bike of the atmospheric water vapor monthly average δ18O, δ17O (A), d-excess (B), relative humidity (C), and temperature (D).
δ18O, δ17O, and d-backlog varied, which is the opposite to temperature changes, and their correlation with humidity (r 2 = 0.81, r 2 = 0.eighty, r 2 = 0.98, and p < 0.001, respectively) is significantly college than temperature (r 2 = 0.81, r two = 0.80, r 2 = 0.96, and p < 0.001, respectively) (Figure 8).
FIGURE viii
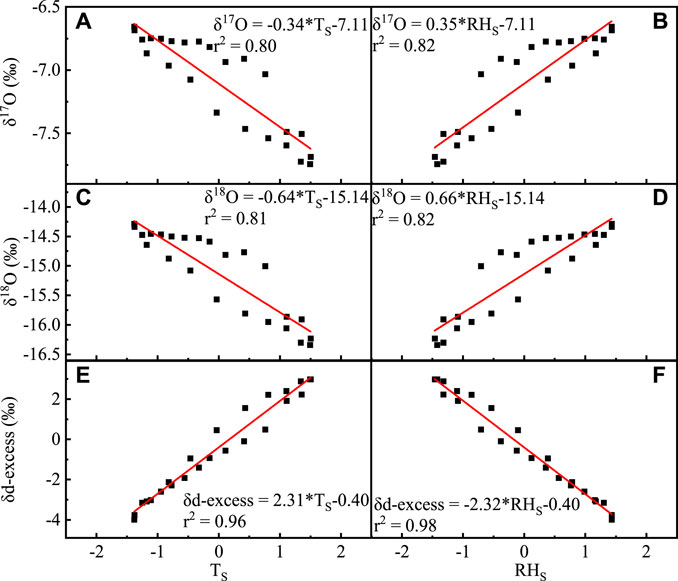
Figure 8. Correlation betwixt monthly boilerplate δ17O, δ18O, and d-excess of the atmospheric water vapor and temperature (A,C,E), and relative humidity (B,D,1F) (TS stands for dimensionless standard treatment of the atmospheric water vapor temperature and RHSouth stands for dimensionless standard treatment of relative humidity).
Changes in Water Vapor Earlier and After Precipitation
Combined with the measured meteorological data, the atmospheric h2o vapor hydrogen and oxygen isotopes within five h after precipitation were retained and compared with the daily boilerplate data for each month. During the observation period, there were five precipitation events (Figure 4). The measured data were derived from the 27 May, 14–xv June, 26–27 June, seven—nine July, 12 September, sixteen September, and 24 Oct fourth dimension periods. During the precipitation menstruation, δ2H, δ17O, and δ18O were relatively consequent, which is approximately reflected in a downwardly tendency, and its value is lower than that in the period of no atmospheric precipitation. Even so, the d-excess value shows an upwardly trend. This demonstrates that the alter in d-excess has a stronger relationship with the modify in relative humidity. This is because when a precipitation event occurs, the principal source of precipitation is the condensation and decrease in high-level water vapor, and the decreasing water vapor evaporates to the near-surface water vapor, resulting in a relatively depression hydrogen-to-oxygen-stable isotope ratio.
The d-excess value in atmospheric water vapor increased, and the δ18O and δ2H values decreased when a precipitation event occurred (Figure 9). In Figures 9B,C, the δeighteenO and δ2H values have different trends during the two atmospheric precipitation periods. When comparing the meteorological conditions of API and APII, there is continued precipitation in API, and the alter in the mean value of δ18O from PI to API reduced from −3.51‰ to −17.14‰, and the hateful value of δ2H decreased from −129.72‰ to −155.87‰. Nevertheless, no precipitation event occurred in APII, and the change in the mean value of δ18O from PII to APII increased from −17.66‰ to −16.11‰, and the mean value of δ2H dropped from −157.22‰ to −140.eighteen‰. This indicates that the more the atmospheric precipitation, the lower the stable isotope of atmospheric h2o vapor, and reflects the effect of precipitation (Zhao et al., 2012).
Effigy 9
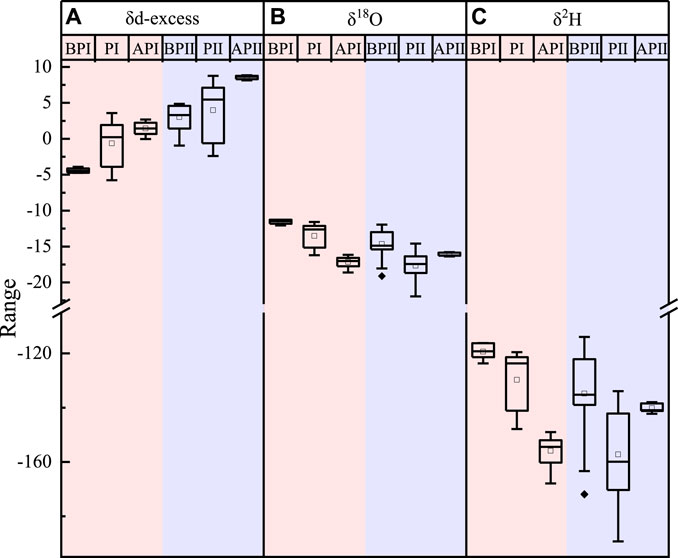
FIGURE nine. Variation trends of d-excess (A), δ18O (B), and δtwoH (C) in the atmospheric water vapor before and after the precipitation effect. BP represents the atmospheric water vapor isotope of the day before the precipitation event, P represents the time of the main precipitation event, AP represents one day after the precipitation event, I represents the big precipitation event that occurred on 26 June 2019, and II represents the large atmospheric precipitation event that occurred on 12 September 2019.
Sources of Water Vapor in Lanzhou Valley Rainfall Period
Previous studies (Vocal and Zhang, 2003; Zhao et al., 2020) have shown that compared to that in other regions in Prc, the distribution of the atmospheric water vapor sources in northwestern arid and semiarid Mainland china is more complicated. It can be divided approximately into three parts, namely, the primal and western parts of the northwest that primarily affected by the westerly zone, the Tibetan plateau h2o vapor areas in Qinghai Province and the Qilian Mountains, and the eastern part of the northwest which is affected by the Asian monsoon fringe. The main current of air direction in May, Oct, and Nov is northeast (36.25, 30.xix, and 36.46%, respectively). In June, the air current management is easterly (23.02%), and a northerly direction (xvi.92%) deemed for the principal influencing factors. The main wind management in July and September is easterly (29.27 and 27.35%, respectively), and the northeasterly wind (24.88%) has a more marked impact in September (Figure 10). Here, the NCEP/NCAP reanalysis and related datasets were used to analyze the wind field data from May to July 2019 and September to November 2019 (Figure 11). From May to July, the wind direction is primarily northwest and southeast, and from September to November, the chief current of air direction is southwesterly and southeasterly. The difference between the measured data and the NCEP/NCAP reanalysis information is considering of the influence of local fine-scale topography. By comparison the isotope data of different current of air directions, it is institute that the summer monsoon water vapor isotope changes are more abundant than winter monsoon and local evaporative water vapor isotopes.
Figure 10
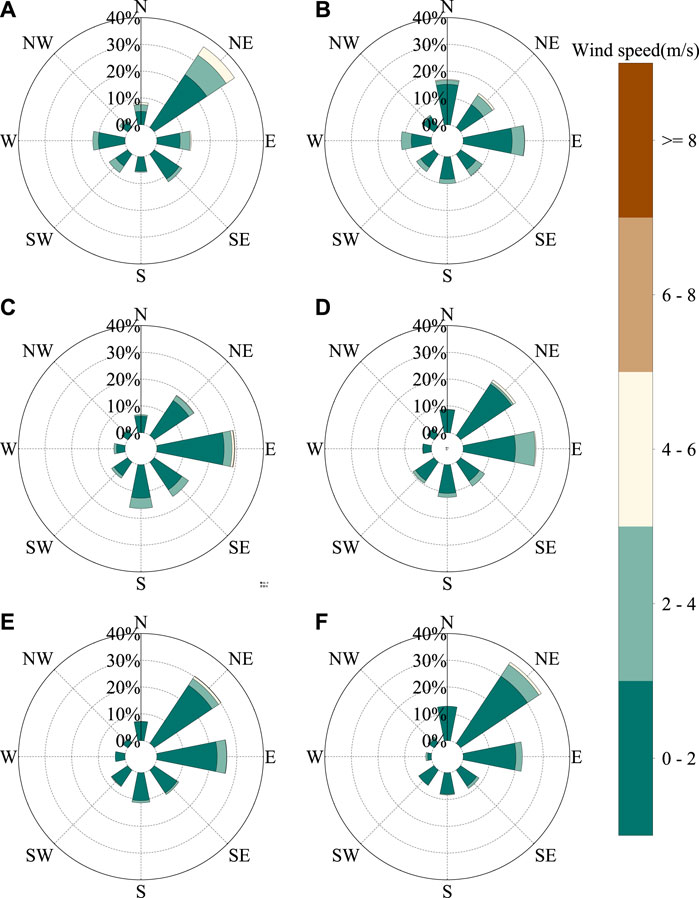
Figure 10. Observation site (Lanzhou valley) current of air rose in 2019 for (A) May, (B) June, (C) July, (D) September, (Due east) October, and (F) Nov.
FIGURE xi
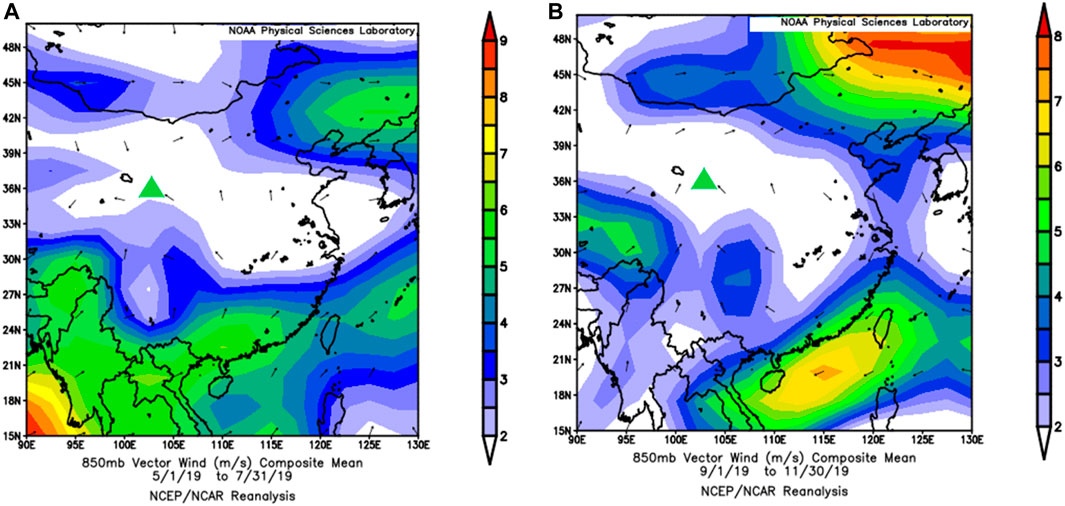
Figure 11. NCEP/NCAP 850 hPa air current field reanalysis dataset for (A) May to July and (B) September to November (green triangles represent study sites).
According to the hybrid unmarried-particle Lagrangian integrated trajectory model (HYSPLIT) adult by the Atmospheric Resources Laboratory of the National Oceanic and Atmospheric Administration and GDAS meteorological data, the operating conditions of the atmospheric water vapor during the observation menstruum are reversed. H2o vapor can remain in the stratosphere for a maximum menstruum of approximately x days, and therefore, the atmospheric water vapor trajectories at different altitudes 10 days earlier the end of the ascertainment (Gat, 2000) were calculated. Three layers of air masses at 1500 g (850 hPa), 3,000 m (700 hPa), and 5,500 thou (500 hPa) were set up (Li et al., 2012), and the observation betoken where the precipitation event occurred was taken every bit the cease point of the atmospheric water vapor mass movement. The 27 May, 15 June, 27 June, 9 July, 12 September, 16 September, 23 October, and 5 November when precipitation events occurred were selected as the start times of the atmospheric h2o vapor inversion.
From May to July, it was primarily afflicted past the westerly water vapor. Water vapor from the Indian Ocean affected atmospheric precipitation events on 27 May and 27 June. The air mass movement of 1500 chiliad is affected past the terrain before reaching the study surface area, and the trajectory motion changes significantly. The air mass of 3,000 m has a short movement altitude. During the movement, the altitude fluctuates frequently, primarily in the interior of the continent. The air mass at five,500 m has a longer distance, primarily from the Atlantic and Indian oceans (Figures 12A–D). From September to November, 1500 m and 5,500 m are primarily affected past the westerly water vapor. In the precipitation effect on 24 October, the movement of the air masses at 3,000 m and v,500 m was afflicted by westerly water vapor, whereas the motion of the air mass at 1500 m was more afflicted by terrain. The h2o vapor sources three,000 one thousand are primarily concentrated in the southwest, east, and west (Figures 12E–H).
FIGURE 12
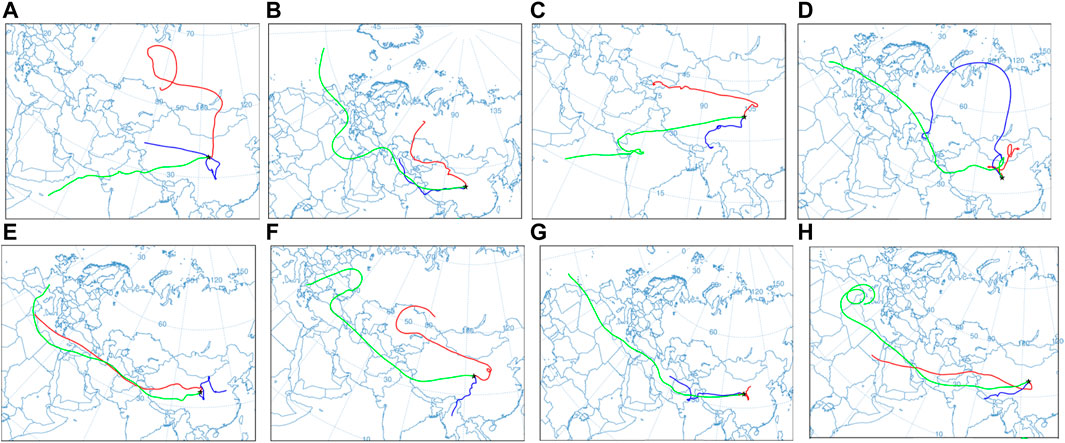
Effigy 12. Astern trajectory clustering of the water vapor in Lanzhou during the 2019 precipitation flow. The blackness 5-pointed stars represent sampling points, the blood-red line represents 1500 1000 air mass trajectories, the green line represents three,000 m air mass trajectories, and the blue line represents 3,300 m air mass trajectories. Conditions on (A) 24 May, (B) xv June, (C) 27 June, (D) 9 July, (E) 12 September, (F) sixteen September, (G) 24 October, and (H) v November.
The atmospheric water vapor isotope values of Lanzhou and Lhasa [29°39′ North, 91°02′ E (Tian et al., 2019)] were compared (Table 2). Lanzhou has a higher δ18O, δ2H, and d-backlog values than Lhasa, except for δ2H in May. Among them, the divergence between δ18O and δ2H is the largest in July and September. Lanzhou is primarily affected past the westerly h2o vapor from the Atlantic Ocean. Lhasa has two sources of water vapor during the monsoon season, namely, water vapor from the Bay of Bengal, and water vapor from the southwest originating from the Arabian Body of water, and dominated by the southern water vapor. This acquired the difference in the atmospheric h2o vapor isotopes between Lanzhou and Lhasa.
Table ii
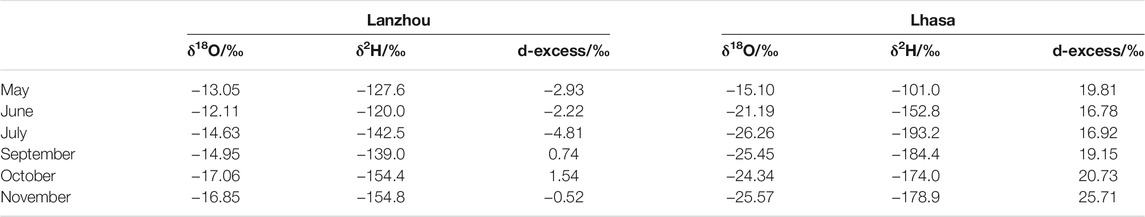
TABLE 2. Comparison of the monthly average atmospheric water vapor isotope values of Lanzhou and Lhasa.
In full general, the ascertainment period is primarily affected by the westerly water vapor, supplemented by local h2o vapor and southwest water vapor. This is consistent with the results of previous studies (Wang et al., 2005; Zhang et al., 2007; Yao et al., 2009; Shi et al., 2015).
Meteoric Waterline in Summertime 2019
Craig (1961) proposed the post-obit global meteoric water line (Craig equation):
This promoted the study of the correlation between stable isotopes of atmospheric precipitation and various meteorological elements. Due to differences in factors affecting stable isotope fractionation from the source of h2o vapor to the fall of raindrops, local meteoric water lines showroom different slopes and intercepts (Figure thirteen). In 1983 Zheng et al. (1983) analyzed the isotope data of the precipitation in different locations in Prc and obtained the following equation for the countrywide atmospheric precipitation line:
Figure xiii
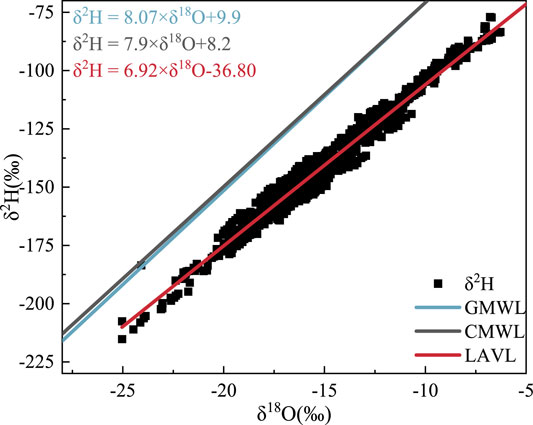
FIGURE 13. Local atmospheric water vapor line of this study. The blood-red line is the atmospheric vapor line fitted by Lanzhou atmospheric water vapor δ18O and δ2H, the black line is Zheng Shuhui'due south Communist china meteoric water line, and the blueish line is Gourcy's global meteoric water line.
The LAVL composed of the atmospheric water vapor δ2H and δeighteenO from May to July and September to November 2019 in Lanzhou city (Figure thirteen) is as follows:
The slope is less than eight, indicating that the sources of the atmospheric h2o vapor in this area have unlike stable oxygen isotope ratios and too reflecting that the h2o vapor has undergone a certain evaporation procedure (Liu et al., 2018). In improver, after the water vapor changes from liquid to gas, and the evaporation fractionation rate of light isotopes is faster than that of heavy isotopes. The smaller the slope and intercept, the stronger the evaporation of the atmospheric water vapor, implying that the secondary evaporation of water vapor nether clouds in the temper produces a strong isotope dynamic fractionation effect, which makes the slope and intercept of the h2o vapor (Peng et al., 2010). When combining Figures xi, 12, the air masses arriving in the report area are divided into two scenarios. One is that water vapor from the Atlantic Ocean arrives across the European continent with the westerly circulation, and the other is the local air mass movement. Moreover, the study area was far from the ocean and river valley topography. This caused the water vapor arriving in the report area to develop sub-deject secondary evaporation, and the slope and intercept of the LAVL were lower than the national level.
Determination
In this written report, we investigated the temporal variations of δ2H, δ18O, δ17O, and d-excess of the atmospheric water vapor from May to November 2019 in Lanzhou. The major findings are summarized as follows:
1) δtwoH, δ17O, δ18O, and d-backlog have a daily cycle change trend betwixt the daily hydrogen and oxygen stable isotopes.
2) The δ17O and δ18O of Lanzhou in May–July and September–November 2019 are positively correlated with temperature, and the correlation of δ17O is weaker than that of δ18O, reflecting the temperature event.
3) During the period of atmospheric precipitation, δtwoH, δ17O, and δ18O in the atmospheric water vapor change uniformly, and the overall trend is downward. Compared with the no atmospheric precipitation period, the value is lower, and temperature dependence is higher than humidity. In that location is a generally consistent and significant correlation between d-excess and temperature and absolute humidity.
4) During the observation catamenia, the atmospheric water vapor in Lanzhou was affected by westerly water vapor, southwesterly water vapor, and local water vapor, of which westerly water vapor was the main influence. Afflicted by local topography, the air current direction is primarily northeast.
5) The LAVL obtained in this study is similar to the atmospheric waterline obtained by Zheng et al. (1983). Due to the sub-deject secondary evaporation and the influence of topography, the gradient and intercept are lower than the national level.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary fabric, further inquiries tin be directed to the corresponding author.
Author Contributions
JL led the formulation and blueprint of the paper, collected, and analyzed data. JL and JD wrote the manuscript. All authors contributed to the development of ideas and manuscript revision and canonical the submitted version.
Funding
This report is supported by the National Scientific discipline Foundation of China (41801054 and 42071047).
Conflict of Interest
The authors declare that the enquiry was conducted in the absence of whatever commercial or financial relationships that could exist construed every bit a potential conflict of interest.
Publisher'due south Note
All claims expressed in this article are solely those of the authors and do not necessarily stand for those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may exist evaluated in this article, or claim that may be made by its manufacturer, is non guaranteed or endorsed by the publisher.
References
Aemisegger, F., Sturm, P., Graf, P., Sodemann, H., Pfahl, S., Knohl, A., et al. (2012). Measuring Variations of δ18O and δ2H in Atmospheric Water Vapour Using Two Commercial Laser-Based Spectrometers: an Instrument Characterisation Report. Atmos. Meas. Tech. 5 (vii), 1491–1511. doi:10.5194/amt-v-1491-2012
CrossRef Full Text | Google Scholar
Angert, A., Lee, J.-Due east., and Yakir, D. (2008). Seasonal Variations in the Isotopic Composition of Near-Surface Water Vapour in the Eastern Mediterranean. Tellus B sixty (four), 674–684. doi:ten.1111/j.1600-0889.2008.00357.10
CrossRef Full Text | Google Scholar
Bailey, A., Noone, D., Berkelhammer, Chiliad., Steen-Larsen, H. C., and Sato, P. (2015). The Stability and Calibration of H2o Vapor Isotope Ratio Measurements during Long-Term Deployments. Atmos. Meas. Tech. eight (10), 5425–5466. doi:10.5194/amt-8-4521-2015
CrossRef Full Text | Google Scholar
Bolin, B. (1959). "On the Employ of Tritium equally a Tracer for Water in Nature," in Proc. 2nd U.N. Conf. Peaceful Uses of Atomic Energy, Oct 31, 1959.
Google Scholar
Chen, F., Zhang, M., Ma, Q., Li, X., Wang, Due south., and Li, F. (2013). Characteristics of δeighteenO in Precipitation and H2o Vapor Sources in Lanzhou Urban center and its Surrounding Area. Environ. Sci. 34, 3755–3763.
Google Scholar
Christner, E., Aemisegger, F., Pfahl, S., Werner, M., Cauquoin, A., Schneider, 1000., et al. (2018). The Climatological Impacts of Continental Surface Evaporation, Rainout, and Subcloud Processes on δ D of Water Vapor and Precipitation in Europe. J. Geophys. Res. Atmos. 123, 4390–4409. doi:10.1002/2017jd027260
CrossRef Total Text | Google Scholar
Craig, H., and Gordon, L. I. (1965). "Deuterium and Oxygen 18 Variations in the Ocean and the marine Atmosphere," in Stable Isotopes in Oceanographic Studies and Palaeotemperatures Editor Due east. Tongiorgi, 9–103.
Google Scholar
Dansgaard, W. (1953). The Affluence of O18in Atmospheric Water and Water Vapour. Tellus 5, 461–469. doi:10.3402/tellusa.v5i4.8697
CrossRef Total Text | Google Scholar
Dong, Ten., Yang, H., Zhang, Fifty., Zhu, Z., Yang, Y., Zheng, X., et al. (2017). Influence of ENSO Events on the Hydrogen (δ2H) and Oxygen (δ18O) Isotopic Values of Precipitation in Shanghai. Environ. Sci. 38, 4991–5003. doi:10.13227/j.hjkx.201705190
PubMed Abstract | CrossRef Full Text | Google Scholar
Eriksson, E. (1965). Deuterium and Oxygen–18 in Precipitation and Other Natural Waters Some Theoretical Considerations 1. Tellus 17 (4), 498–512. doi:10.1111/j.2153-3490.1965.tb00212.ten
CrossRef Full Text | Google Scholar
Galewsky, J., Steen-Larsen, H. C., Field, R. D., Worden, J., Risi, C., and Schneider, M. (2016). Stable Isotopes in Atmospheric Water Vapor and Applications to the Hydrologic Wheel. Rev. Geophys. 54, 809–865. doi:x.1002/2015rg000512
PubMed Abstract | CrossRef Full Text | Google Scholar
Gat, J.R. (2000). Atmospheric Water Residuum—The Isotopic Perspective. Hydrol. Process. 14 (8), 1357–1369. doi:10.1002/1099-1085(20000615)14:viii<1357::AIDHYP986>3.0.CO;2-7
CrossRef Full Text | Google Scholar
Gourcy, L. Fifty., Gröning, 1000., and Aggarwal, P. (2005). Stable Oxygen and Hydrogen Isotopes in Precipitation. Dordrecht: Springer, 39–51. doi:10.1007/one-4020-3023-1_4
CrossRef Full Text | Google Scholar
Gu, X., Pang, H., Li, Y., Zhang, W., and Wang, J. (2019). Written report on Scale Method for Atmospheric H2o Vapor Stable Isotopes Observed by Crenel Ring-Down Spectroscopy. Spectrosc. Spectral Anal. 039, 1700–1705. doi:ten.3964/j.issn.1000-0593(2019)06-1700-06
CrossRef Full Text | Google Scholar
Huang, L., and Wen, X. (2015). Temporal Variations of Atmospheric H2o Vapor δD and δ18O above an Arid Artificial Oasis Cropland in the Heihe River Basin. J. Geophys. Res. Atmospheres 119, 11,456–411,476. doi:10.1002/2014JD021891
CrossRef Full Text | Google Scholar
He, J., Pang, H., and Hou, S. (2015). Progress in the Study of 17O-Backlog in Snow and Water ice over Polar Regions. Polar Res., 392–401. doi:10.13679/j.jdyj.2015.4.392
CrossRef Full Text | Google Scholar
Kurita, N., Hirasawa, N., Koga, S., Matsushita, J., Steen-Larsen, H. C., Masson-Delmotte, 5., et al. (2016). Influence of Large-Scale Atmospheric Apportionment on Marine Air Intrusion Toward the East Antarctic Declension. Geophys. Res. Lett. 43 (17), 9298–9305. doi:10.1002/2016gl070246
CrossRef Full Text | Google Scholar
Li, J.-l., Li, Z.-r., Yang, J.-c., Shi, Y.-z., and Fu, J. (2012). Analyses on Spatial Distribution and Temporal Variation of Temper H2o Vapor Over Northwest China in Summer of Later 10 Years. Plateau Meteorol. 31 (half-dozen), 1574–1581.
Google Scholar
Liu, J. Y., Zhang, F. P., Feng, Q., Li, Z. X., Zhu, Y. W., Nie, South., et al. (2018). Influence of Beneath-Cloud Secondary Evaporation on Stable Isotope Composition in Precipitation in Northwest People's republic of china. Ying Yong Sheng Tai Xue Bao 29, 1479–1488. doi:10.13287/j.1001-9332.201805.028
PubMed Abstract | CrossRef Total Text | Google Scholar
Liu, X., Qin, D., Shao, Ten., Ren, J., and Wang, Y. (2002). Stable Carbon Isotope of Abies Spectabibis from Nyingchi Canton of Tibet Autonomous Region and its Response to Climate Change. J. Glaciology Geocryology 24 (5, 574–578. doi:ten.3969/j.issn.grand-0240.2002.05.018
CrossRef Total Text | Google Scholar
Luz, B., and Barkan, E. (2010). Variations of 17O/16O and 18O/16O in Meteoric Waters - ScienceDirect. Geochimica et Cosmochimica Acta 74, 6276–6286. doi:10.1016/j.gca.2010.08.016
CrossRef Full Text | Google Scholar
Ma, H., Li, Y., Jiang, S., and An, C. (2010). Advance in the Study of Mass Independent Oxygen Isotope Fractionation. World Environ. 38, 91–97. doi:10.3969/j.issn.1672-6561.2010.02.011
CrossRef Full Text | Google Scholar
Merlivat, Fifty., and Jouzel, J. (1979). Global Climatic Interpretation of the Deuterium-Oxygen 18 Relationship for Atmospheric precipitation. J. Geophys. Res. 84, 5029–5033. doi:10.1029/jc084ic08p05029
CrossRef Full Text | Google Scholar
Pang, H., Hou, S., Landais, A., Masson-Delmotte, Five., Prie, F., Steen-Larsen, H. C., et al. (2015). Spatial Distribution of 17O-Backlog in Surface Snow along a Traverse from Zhongshan Station to Dome A, East Antarctica. Earth Planet. Sci. Lett. 414, 126–133. doi:10.1016/j.epsl.2015.01.014
CrossRef Full Text | Google Scholar
Peng, H., Mayer, B., Harris, Due south., and Krouse, H. R. (2010). A ten-twelvemonth Tape of Stable Isotope Ratios of Hydrogen and Oxygen in Atmospheric precipitation at Calgary, Alberta, Canada. Tellus 56, 147–159. doi:10.1111/j.1600-0889.2004.00094.x
CrossRef Full Text | Google Scholar
Ren, Due west., Yao, T., Xie, S., and He, Y. (2016). Controls on the Stable Isotopes in Precipitation and Surface Waters across the southeastern Tibetan Plateau. J. Hydrol. 545, 276–287. doi:ten.1016/j.jhydrol.2016.12.034
CrossRef Full Text | Google Scholar
Rozanski, K., and Sonntag, C. (1982). Vertical Distribution of Deuterium in Atmospheric Water Vapour. Tellus 34 (2), 135–141. doi:10.3402/tellusa.v34i2.10795
CrossRef Full Text | Google Scholar
Rozanski, K., Araguás-Araguás, 50., and Gonfiantini, R. (1992). Relation between Long-Term Trends of Oxygen-18 Isotope Composition of Precipitation and Climate. Science 258, 981–985. doi:10.1126/scientific discipline.258.5084.981
PubMed Abstract | CrossRef Full Text | Google Scholar
Shi, G., Buffen, A. M., Hastings, M. G., Li, C., Ma, H., Li, Y., et al. (2015). Investigation of Post-Depositional Processing of Nitrate in Due east Antarctic Snow: Isotopic Constraints on Photolytic Loss, Re-oxidation, and Source Inputs. Atmos. Chem. Phys. 15, 9435–9453. doi:10.5194/acp-15-9435-2015
CrossRef Full Text | Google Scholar
Song, L., and Zhang, C. (2003). Changing Features of Precipitation over Nothwest Communist china during the 20th Century. J. Glaciology Geocryology 25, 143–148. doi:x.3969/j.issn.1000-0240.2003.02.005
CrossRef Total Text | Google Scholar
Steen-Larsen, H., Masson‐Delmotte, V., Sjolte, J., Johnsen, S., Vinther, B., Bréon, F.1000., et al. (2011). Agreement the Climatic Signal in the H2o Stable Isotope Records from the NEEM Shallow Firn/Ice Cores in Northwest Greenland. J. Geophys. Res. 116 (D6). doi:10.1029/2010JD014311
CrossRef Full Text | Google Scholar
Steen-Larsen, H. C., Johnsen, S. J., Masson-Delmotte, 5., Stenni, B., and White, J. W. C. (2013). Continuous Monitoring of Summer Surface Water Vapour Isotopic Composition higher up the Greenland Ice Canvas. Atmos. Chem. Phys. 13 (1), 4815–4828. doi:10.5194/acpd-thirteen-1399-2013
CrossRef Total Text | Google Scholar
Steen-Larsen, H. C., Sveinbjörnsdottir, A. E., Peters, A. J., Masson-Delmotte, V., Guishard, M. P., Hsiao, Chiliad., et al. (2014). Climatic Controls on Water Vapor Deuterium Excess in the marine Boundary Layer of the Due north Atlantic Based on 500 Days of In Situ, Continuous Measurements. Atmos. Chem. Phys. xiv, 7741–7756. doi:10.5194/acp-14-7741-2014
CrossRef Total Text | Google Scholar
Tian, L., Yao, T., Yang, Z., and Pu, J. (1997). A 4-twelvemonth'due south Study of Oxygeo Isotope in Atmospheric precipitation at Tuotuohe Meteorological Station, Tibetan Plateau. J. Glaciol. Geocryol. twenty (4), 438–443.
Google Scholar
Tian, Fifty., Yu, West., Schuster, P. F., Wen, R., Cai, Z., Wang, D., et al. (2019). Control of Seasonal Water Vapor Isotope Variations at Lhasa, Southern Tibetan Plateau. J. Hydrol. 580, 124237. doi:10.1016/j.jhydrol.2019.124237
CrossRef Full Text | Google Scholar
Vuille, M., Werner, M., Bradley, R. S., and Keimig, F. (2005). Stable Isotopes in Atmospheric precipitation in the Asian Monsoon Region. J. Geophys. Res. 110 (D23108), 1–15. doi:10.1029/2005JD006022
CrossRef Full Text | Google Scholar
Wang, K., Jiang, H., and Zhao, H. (2005). Atmospheric Water Vapor Transport from Westerly and Monsoon Over the Northwest Cathay. Adv. Water Sci. 16, 432–438. doi:x.14042/j.cnki.32.1309.2005.03.021
CrossRef Total Text | Google Scholar
Welp, L. R., Lee, X., Kim, Thousand., Griffis, T. J., Billmark, K. A., and Baker, J. M. (2008). deltaO of Water Vapour, Evapotranspiration and the Sites of Leaf Water Evaporation in a Soybean Canopy. PLANT CELL ENVIRON. 31 (ix), 1214–1228. doi:10.1111/j.1365-3040.2008.01826.x
PubMed Abstruse | CrossRef Total Text | Google Scholar
Wen, X. F., Zhang, S. C., Sun, X. 1000., Yu, G. R., and Lee, Ten. (2010). Water Vapor and Precipitation Isotope Ratios in Beijing, Mainland china. J. Geophys. Res. Atmospheres 115 (D01103), 1–10. doi:10.1029/2009jd012408
CrossRef Total Text | Google Scholar
Xie, Y. 50., Zhang, X. P., Yao, T. C., and Huang, H. (2016). Monitoring and Assay of Stable Isotopes of the Near Surface Water Vapor in Changsha. Huan Jing Ke Xue 37, 475–481. doi:10.13227/j.hjkx.2016.02.010
PubMed Abstruse | CrossRef Full Text | Google Scholar
Yao, T., Zhou, Ten., and Yang, 10. (2009). Indian Monsoon Influences Altitude Issue of δ 18O in Precipitation/River Water on the Tibetan Plateau. Chin. Sci. Bull. 54, 2124–2130. doi:10.1007/s11434-009-0497-4
CrossRef Total Text | Google Scholar
Zhang, L., Zhao, Q., Zhang, M., Guo, J., Zheng, J., Chen, Z., et al. (2020). Mg2+ Distribution in Activated Sludge and its Effects on the Nitrifying Activity and the Characteristics of Extracellular Polymeric Substances and Sludge Flocs. Process Biochem. 88, 120–128. doi:ten.1016/j.procbio.2019.10.002
CrossRef Full Text | Google Scholar
Zhang, Q., Zhang, J., Song, Chiliad., and Di, Ten. (2007). Research on Atmospheric Wayer-Vapor Distribution over Qilianshan Mountains. Acta Meteorologica Sinica 65, 633–643. doi:10.3321/j.issn:0577-6619.2007.04.015
CrossRef Full Text | Google Scholar
Zhang, X., and Yao, T. (1998). Distributional Features of δ18O in Precipitation in Mainland china. Acta Geographica Sinica 53, 70–78. doi:10.11821/xb199804008
CrossRef Full Text | Google Scholar
Zhang, 10., Yao, T., Liu, J., Tian, L., and Masayoshi, N. (2003). Simulations of Stable Isotopic Fractionation in Mixed Cloud in Middle Latitudes-Taking the Precipitation at Ürümqi as an Example. Adv. Atmos. Sci. twenty, 261–268. doi:ten.1007/s00376-003-0012-nine
CrossRef Full Text | Google Scholar
Zhao, L., Xiao, H., Zhou, M., Cheng, One thousand., Wang, L., Yin, 50., et al. (2012). Factors Controlling Spatial and Seasonal Distributions of Atmospheric precipitation δ18O in Cathay. Hydrol. Process. 26, 143–152. doi:10.1002/hyp.8118
CrossRef Total Text | Google Scholar
Zhao, P. (2018). Stable Isotopic Characteristics and Influencing Factors in Precipitation in the Monsoon Region of Northern China. Xi'an China: Northwest University.
Google Scholar
Zhao, Q., Chuancheng, Z., Qin, Y., and Chang, Y. (2020). The Alter Features and Future Tendency of Snowfall and Extreme Snowfall in the Arid Areas of Northwest People's republic of china. J. Glaciology Geocryology 42, 81–xc. doi:x.7522/j.issn.m-0240.2020.0025
CrossRef Total Text | Google Scholar
Zhao, S. (1983). A New Scheme for Comprehensive Physical Regionalization in China. Acta Geographica Sinica 38, ane–10. doi:ten.11821/xb198301001
CrossRef Total Text | Google Scholar
Zheng, S., Hou, F., and Ni, B. (1983). Research on Hydrogen and Oxygen Stable Isotopes of Precipitation in My State. Chin. Sci. Bull. (13), 801.
Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fenvs.2022.843948/full
Posted by: mongerhicis1948.blogspot.com

0 Response to "Monsoon moisture continues and temperature changes for the valley's"
Post a Comment